WE WILL HELP YOU
TO LIVE A HEALTHY LIFE


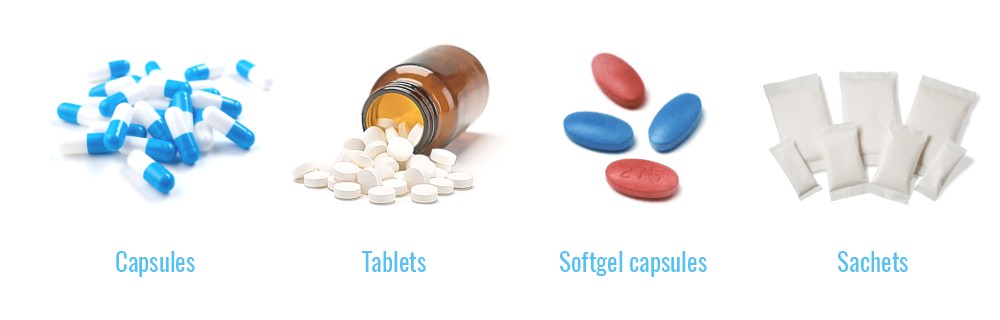
products formulation
and production
packaging services
regulatory affairs –
preparation of documents
for the registration process

AN EXTENSION OF YOUR TEAM
At Dox Supplements each, and every one of our team is cross trained and highly educated on all aspects of the private label manufacturing process. We work collaboratively to find the perfect solution for your business. We take this collaborative approach with our clients, giving them the opportunity to get involved at every stage of the process.

THE HIGHEST QUALITY STANDARDS
Every raw material that enters our plant is tested to ensure it meets our highest standards. We carry out additional rigorous testing throughout the manufacturing process ensuring the safety and efficacy of every product that we produce. Prior to leaving our facility, all products are sent for an independent analysis and the completed CoA is provided with every order.
We take safety very seriously, with lot numbers printed on every product for audit security and safety verification.


OUR NETWORK
We have a large business network that supports the growing needs of our clients and we are always looking for reliable partners to expand our network.
We are currently exporting to the UK, Europe, USA and ASIA. Get in touch to find what we can do to maximize your growth and profitability.
WE GUARANTEE
Here at Dox Supplements we are committed to providing high quality products and outstanding customer service. We believe our growing customer base provides testament to this.

Best available quality of products and raw materials

Providing help with the development of new products

Respecting your needs saving your time and money

We will do everything we can to meet or better your deadlines

Best pricing for your private label custom products

Covid-19 pandemics – protective material
A new virus called the severe acute respiratory syndrome coronavirus 2 has been identified as the cause of a disease outbreak that began in China in 2019. The disease is called coronavirus disease 2019 (COVID-19). Cases of COVID-19 have been reported in a growing number of countries, WHO declared a global pandemic in March 2020. Public health groups, such as the World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC), are monitoring the situation and posting updates, treatment and prevention recommendations on their websites.
There is currently no vaccine to prevent 2019-nCoV infection. The best way to prevent infection is to avoid being exposed to this virus. However, as a reminder, CDC always recommends everyday preventive actions to help prevent the spread of respiratory viruses, including:
- Wash your hands often with soap and water for at least 20 seconds. If soap and water are not available, use an alcohol-based hand sanitizer.
- Avoid touching your eyes, nose, and mouth with unwashed hands.
- Avoid close contact with people who are sick.
- Stay home when you are sick.
- Cover your cough or sneeze with a tissue, then throw the tissue in the trash.
- Clean and disinfect frequently touched objects and surfaces
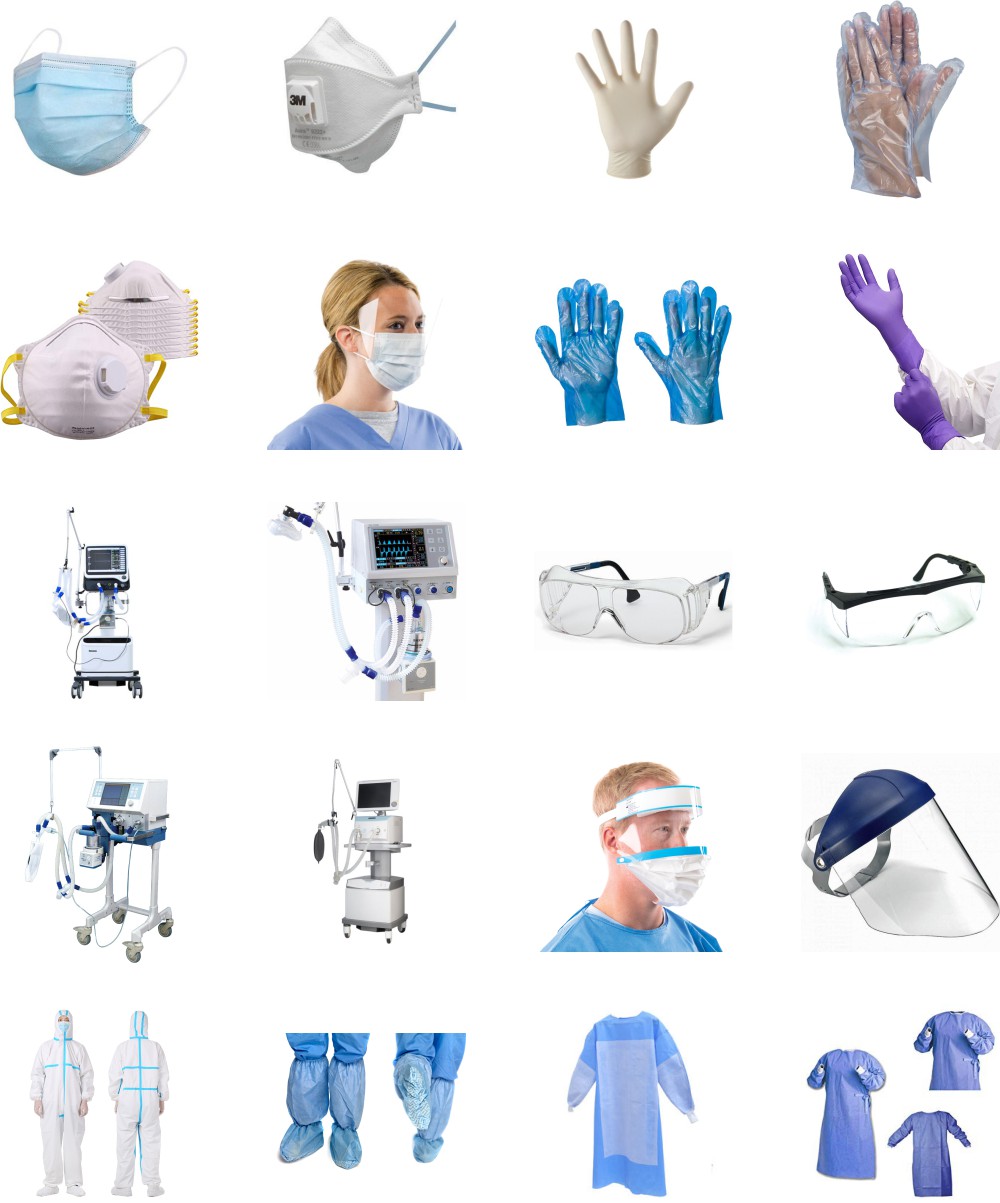
- Wear protective clothing, include masks, gloves, gowns, and eye protection
- Support your health and boost your immune system
- Try to stay calm
Personal Protective Equipment (PPE) should be worn as advised for infection prevention and control in healthcare settings.
OPEN DOCUMENT
Personal Protective Equipment (PPE) should be:
- compliant with the relevant BS/EN standards
- located close to the point of use;
- stored to prevent contamination in a clean/dry area until required for use (expiry dates must be adhered to);
- single-use only;
- changed immediately after each patient and/or following completion of a procedure or task;
- disposed of after use into the correct waste stream i.e. healthcare/clinical waste
Classification of Personal Protection Equipment:
OPEN DOCUMENT 1OPEN DOCUMENT 2
How to properly putt on Protective Equipment (PPE):
Remember
- PPE should be put on and removed in an order that minimises the potential for cross-contamination.
- The order for PPE removal is gloves, apron or gown, eye protection, surgical face mask or FFP3 respirator.
- Hand hygiene must always be performed following removal of PPE.
- Healthcare workers who have had influenza vaccination, or confirmed influenza infection, are still advised to use the above infection control precautions.
CONTACT

Deborah Verdon
Sales and Business manager
Dox supplements
Dox Supplements
Prvoslava Rakovića 12
Kragujevac
Serbia
Deborah Verdon
Sales and Business manager
Dox supplements